The development of the cochlear implant-based gene electrotransfer capability which is central to the CINGT program has built from basic and applied research in neuroscience and biomedical engineering. The following are links to studies and commentary that are in the public domain.
* Close-Field Electroporation Gene Delivery Using the Cochlear Implant Electrode Array Enhances the Bionic Ear
* Close-Field Electroporation Gene Delivery Using the Cochlear Implant Electrode Array Enhances the Bionic Ear
Jeremy L. Pinyon, Sherif F. Tadros, Kristina E. Froud, Ann C. Y. Wong, Isabella T. Tompson, Edward N. Crawford, Myungseo Ko, Renée Morris, Matthias Klugmann, Gary D. Housley
Science Translational Medicine23 Apr 2014 : 233ra54
Neurotrophin gene delivery, achieved by “close-field” electroporation (gene electrotransfer) of mesenchymal cells adjacent to the cochlear implant, improved hearing in deafened guinea pigs.
Commentary: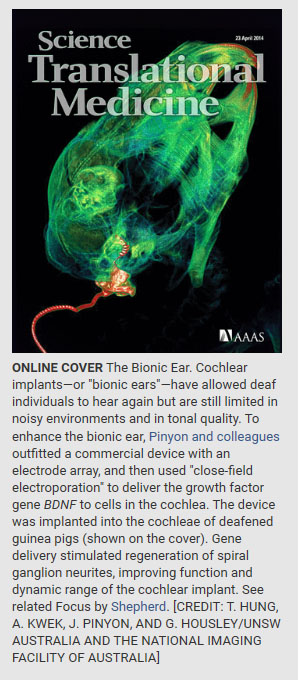
Robert K. Shepherd, Andrew K. Wise Science Translational Medicine23 Apr 2014 : 233fs17
Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear (Pinyon et al., this issue).
The Naked Scientists - "Improving the cochlear implant"- an interview with Gary Housley, University of New South Wales 28th April, 2014.
Click here to download the audio file
* "Mapping of bionic array electric field focusing in plasmid DNA-based gene electrotransfer"
Browne CJ, Pinyon JL, Housley DM, Crawford EN, Lovell NH, Klugmann M, Housley GD (2016) Gene Ther 23:369–379. doi:10.1038/gt.2016.8
* "Cochlear Implant Close-Field Electroporation"
Gary D. Housley, Cherylea J. Browne, Edward N. Crawford, Matthias Klugmann, Nigel H. Lovell, Jeremy L. Pinyon. In: Miklavcic D. (eds) Handbook of Electroporation. Springer, Cham (2016). pp. 1-20. DOI: https://doi.org/10.1007/978-3-319-26779-1_59-1.
Jeremy L Pinyon, Matthias Klugmann, Nigel H. Lovell, Gary D Housley (2018). Human Gene Therapy 30(2). Published Online: 8 Feb 2019 https://doi.org/10.1089/hum.2018.062
* "Neurotrophin gene augmentation by electrotransfer to improve cochlear implant hearing outcomes".
Jeremy L Pinyon, von Jonquieres G, Crawford EN, Duxbury M, Al Abed A, Lovell NH, Klugmann M, Wise AK, Fallon JB, Shepherd RK, Birman CS, Lai W, McAlpine D, McMahon C, Carter PM, Enke YL, Patrick JF, Schilder AGM, Marie C, Scherman D, Housley GD. (2019) Hearing Research. 2019 Sep 1;380:137-149. doi: 10.1016/j.heares.2019.06.002Pinyon.
Conflict of Interest
The development of Bionic array Directed Gene Electrotransfer (BaDGE® - copyright holder UNSW Sydney through assignee New South Innovations Pty Ltd) includes a portfolio of patent filings associated with this research. Many of these patent descriptions are in the public domain. These include: (i) Title: Method of providing agents to the cochlea. Inventor: GD Housley. Filing status: National Phase Examination – Europe (application no. 10799287.7; filing date 5 July 2010), United States (application no. 14/145,673; approved 26th August 2016). Assignee: NewSouth Innovations Pty Limited. (ii) Title: Method and apparatus for close-field electroporation. Inventor: GD Housley, M Klugmann, J Pinyon. Filing status: Provisional, Australia (application no. 2013902263; filed 21 June 2013). Assignee: NewSouth Innovations Pty Limited. (iii) Title: Electroporation system for controlled localized therapeutics delivery. Inventor: GD Housley, NH Lovell. Filing status: Australian Provisional Patent filing (application no. 2015902456; filing date 25 June 2015). Assignee: NewSouth Innovations Pty Limited.